FIFARMA launches the second version of the W.A.I.T. Indicator Survey measuring the waiting time to access innovative drugs in LATAM
FIFARMA launches the second version of the W.A.I.T. Indicator Survey measuring the waiting time to access innovative drugs in LATAM
By FIFARMA
Access the full report FIFARMA W.A.I.T. Indicator 2023
February 14, 2024.. The Latin American Federation of the Pharmaceutical Industry (FIFARMA) launched the second version of the FIFARMA W.A.I.T. (Waiting to Access Innovative Therapies) Indicator 2023 for Latin America. This study analyzes the waiting time Latin American patients experience to access innovative drugs for cancer and orphan diseases. The project is inspired by a European initiative by IQVIA for the European Federation of Pharmaceutical Industries and Associations (EFPIA) since 2004.
The survey analyzes the availability and approval times of 228 innovative treatments from their approval by the regulatory agencies of the United States (FDA) and the European Union (EMA) until they become available to patients in eight (8) Latin American countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Mexico, and Peru.
The FIFARMA W.A.I.T. Indicator highlights disparities in Latin American patients' access to medicines. It also reveals that the average waiting time from FDA approval to effective availability for patients in the region ranges from 1.9 to over 4.5 years. It is estimated that an innovative cancer treatment that receives regulatory approval in the United States in February 2024, for example, could be available to Latin American patients in the second half of 2028.
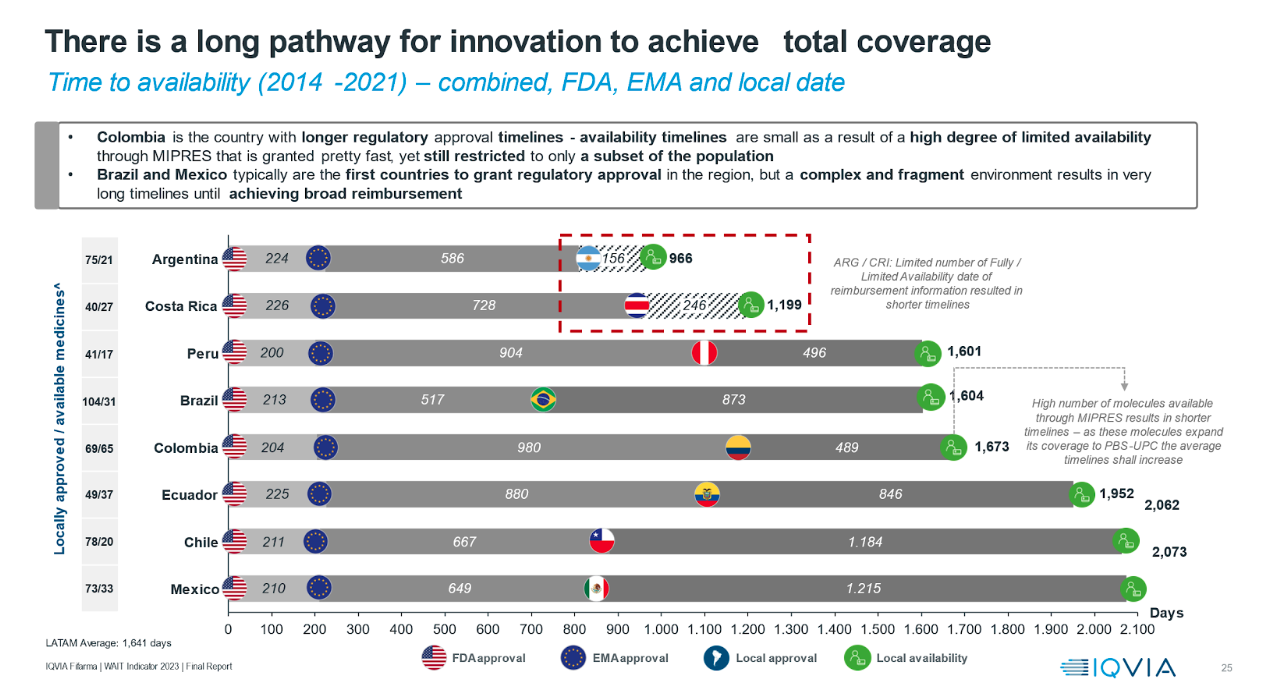
Moreover, the study shows that out of the 228 drugs approved by the EMA and the FDA only 130 have regulatory approval in at least one Latin American country, and only 86 have broad availability in at least one of the analyzed countries.
Regarding these results, Andre Ballalai, access leader at IQVIA Latam, said: “The study reveals that Colombia has the longest regulatory approval times. It also indicates that Brazil and Mexico tend to be the first countries to grant regulatory approval in the region. However, a complex and fragmented environment results in very long lead times to achieve broad reimbursement."
Yaneth Giha, Executive Director at FIFARMA, said: “Our goal is to ensure that Latin American patients have broad and equitable access to innovative medicines. The report highlights the importance of collaboration between the pharmaceutical industry, government agencies, distributors, physicians, and patient associations to improve treatment access. Together, we can offer hope to millions and ensure that health innovations benefit everyone equally.”
This report highlights the delays faced by Latin American patients when trying to access innovative treatments for cancer and rare diseases. It should be noted that the study is not intended to present the reasons behind these delays. It constitutes a starting point to motivate collaboration between the different participants in the regional health system to identify and address the causes of these delays, thus improving patient access to the latest medical and scientific innovations.
Access the full report FIFARMA W.A.I.T. Indicator 2023
Press Contact:
María Alejandra De Guzmán
madeguzman@fifarma.org


